E-pH Diagram
The Environment is one of the key factors in any corrosion situation. The thermodynamics, eg, Pourbix or E-pH diagrams is to evaluate the theoretical activity of a given metal or alloy provided the chemical makeup of the environment is known. For corrosion in aqueous media, two fundamental variables, namely corrosion potential and pH, are deemed to be particularly important. Changes in other variables, such as the oxygen concentration, tend to be reflected by changes in the corrosion potential
Figure 1 illustrates the E-pH diagram for iron in the presence of water or humid environments at 25oC, which was calculated by considering all possible reactions associated with iron in wet or aqueous conditions, which is discussed in corrosion mechanism.
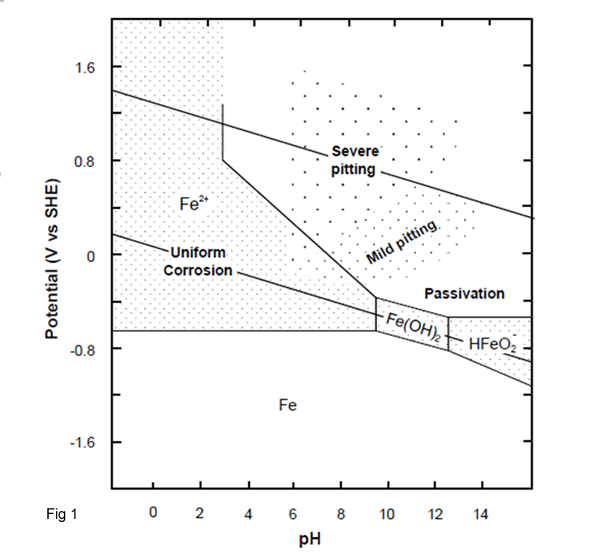
At potentials more positive than about -0.6 and at pH values below about 9, ferrous ion (Fe2+ or Fe II) is the stable substance. This indicates that iron will corrode under these conditions. In other regions of the iron E-pH diagram, it can be seen that the corrosion of iron produces ferric ions (Fe3+ or Fe III), ferric hydroxide [Fe(OH)3], ferrous hydroxide [Fe(OH)2], and at very alkaline conditions, complex HFeO2- ions. The solid corrosion products considered are different than earlier, ferric oxide (Fe2O3) and magnetite (Fe3O4), both important iron ore constituents.

