High temperture Thermodynamic
Oxidation is the most common form of high temperature corrosion – almost all useful metals and alloys will oxidize above a certain temperature, leading to scaling, loss of material and changes in physical properties. Gaseous attack is not limited to oxygen however, with sulphur-bearing gases, carbon oxides, nitrous oxides, halogens and many more all attacking materials in a different manner.
Strictly speaking, high-temperature oxidation is only one type of high-temperature corrosion, but it is the most important high-temperature corrosion reaction. In most industrial environments, oxidation often participates in the high-temperature corrosion reactions, regardless of the predominant mode of corrosion.
Often determination of the conditions under which a given corrosion product is likely to form is required (i.e., in selective oxidation of alloys). The plots of the standard free energy of the reaction (ΔG0) as a function of temperature, commonly called Ellingham diagrams, can help to visualize the relative stability of metals and their oxidized products.
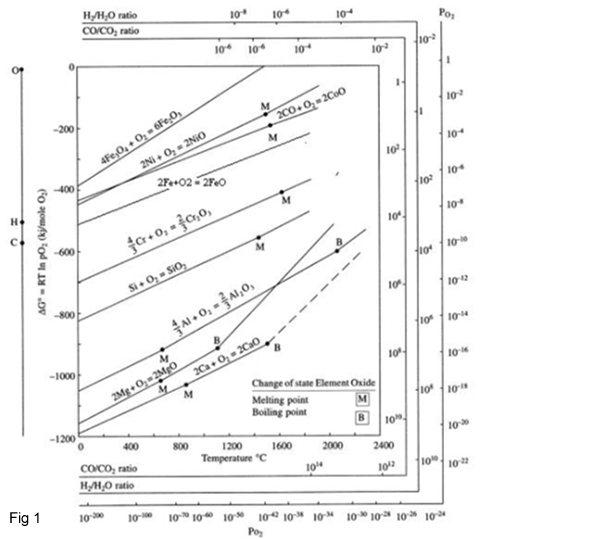
Figure 1 shows an Ellingham diagram for many simple oxides. The values of ΔG0 on an Ellingham diagram are expressed as kilojoules per mole of O2 to normalize the scale and be able to compare the stability of these oxides directly (i.e., the lower the position of the line on the diagram, the more stable is the oxide).
Me + O2 = MeO2
K = aMexPO2/aMeO2
∆G0 = -RTlnK = -RTlnaMe.PO2/aMeO2 = aMe.PO2 By pure material aMe and aMeO2=1
PO2Me/MeO> = e∆G0/RT Or Log PO2Me/MeO2 =∆G0/RT
The values of Po2Me/MeO may be obtained directly from the Ellingham diagram by drawing a straight line from the origin marked O through the free-energy line at the temperature of interest and reading the oxygen pressure from its intersection with the scale at the right side labeled Log(pO2). Values for the pressure ratio H2/H2O for equilibrium between a given metal and oxide may be obtained by drawing a similar line from the point marked H to the scale labelled H2/H2O ratio, and values for the equilibrium CO/CO2 ratio may be obtained by drawing a line from point C to the scale CO/CO2 ratio.