Direct Reduction
The iron ore is typically smelted in Blast furnaces that use primarily iron ore, iron concentrate pellets, metallurgical coke, limestone and lime as the row materials. Under current operation process, the iron produced from the blast furnace is relatively expensive as compared to current alternative direct iron reduction process.
Blast furnace has the following disadvantages:
- Many of the blast furnaces are relatively small, as compared to the newer larger blast furnace, therefore they are relatively costly
- Blast furnace need high –grade metallurgical grade coke
- The cost of cokes is increased because cokes must be produced according to environmental requirements.
Therefore, there is a signicant need to develop or extend the economic viability of alternate Iron-making process. One of type of these processes is Direct Reduction Processes:
Direct reduction process is obtained when fine (pelletized) or lump ore are reduced in solid state at the relatively low temperature of about 900 -1100 oC using reformed natural gas.
Reaction of Reduction process
Most of the row materials in these processes are in the solid state and the reactions with CO at about 900 -1100 oC are according the following reactions:
3Fe2O3 + CO or ( H2) = 2 Fe3O4 +CO2 (H2O)
Fe3O4 + CO or (H2) =3 FeO + CO2 (H2O)
FeO + CO or (H2) = Fe + CO2 (H2O)
If iron oxide is present as a liquid, a second reaction is also possible:
FeO + C = Fe + COCarbon monoxide is produced in the blast furnace air with hot coke and other reducing agents such as injected coal or oil
C + ½ O2 = CO
Carbon monoxide is also the product of the Boudouard reaction
C + CO2 = 2CO
Carbon dioxide required by above reaction is formed during the above reaction as well.In some cases coal is utilized as the reducing agent.
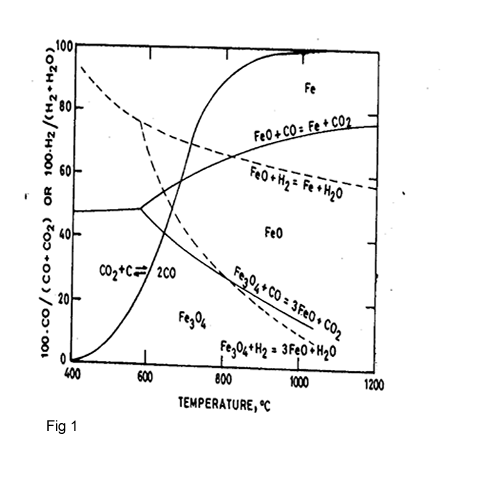
Figure 1 shows that at a temperature over 800 oC the reducibility of hydrogen is bigger than CO, therefore it is possible to use the H2 as reduction agent.
An appropriate gas for reduction above the decomposition temperature of carbon monoxide is that produced by steam reforming, with a composition of 73% H2 and 13 % CO, 1% H2O ,8% CO2 and 5% CH4 according the following reaction:
CH4 + H2O = CO + 3H2
In the Midrex process the ratio of H2/CO = 1.5/1.This ratio will be achieved by reaction of natural gas with blast furnace topgas.
CH4 + CO2 = 2CO + 2H2
Reduction gas should contain as little steam and carbon dioxide as possible. The ratio CO/CO2 or H2/H2O must not fall significantly below 2.3.
Technology of direct reduction
There are many direct reduction process in the industries, but the Midrex and HYL-III processes are now most commonly used for direct reduction. These have been successfully industrialized in large scale production. About 67% of the DRI is produced by Midrex, about 23% of DRI by HYL process and the rest of other processes .
For more information please contact us.

