Midrex
The most successful gas based direct reduction process is the Midrex process. MIDREX is designed to meet current steelmaker needs while being flexible to adapt to tomorrow's markets. Of the 67 MIDREX® Plants built and started-up since 1969, only 3 have ever been dismantled and taken completely out of service. In fact, 33 MIDREX Plants have been operating more than 20 years and some are in their fourth decade of operation. The MIDREX Process can utilize either natural gas or coal with MXCOL to produce quality DRI, HBI and HDRI that provides specialized solutions for steelmakers worldwide in the field of direct reduction ironmaking (DRI). It can use the widest range of oxide pellets and lump and has three hot transport solutions for a variety of site layouts, all of which minimize fines generation. Midrex Corporation is an international process technology company, wholly owned by Kobe Steel, Ltd. The original process was developed by the Midland-Ross Co.
Midrex process
Either lump ore, or pellets prepared for direct reduction ironmaking, are charged as raw material from the top of a shaft furnace (fig 1). The ore is reduced inside the furnace at a temperature ca.780 – 900 0C and the reduced iron is discharged from the bottom of the furnace. Reductant gas blown in from about the middle of the shaft furnace reduces the raw material above the nozzle and escapes from the top of the furnace. The cooling gas, which circulates in the lower portion of the furnace, cools the DRI to ca.45 0C. Both the charging and discharging ports are dynamically sealed by a sealing gas, allowing the continuous charging of raw material and discharging of DRI. The reaction process occurring in the shaft furnace is as follows:
Fe2O3 + 3CO = 2Fe + 3CO2
Fe2O3 + 3H2 = 2Fe + 3H2O
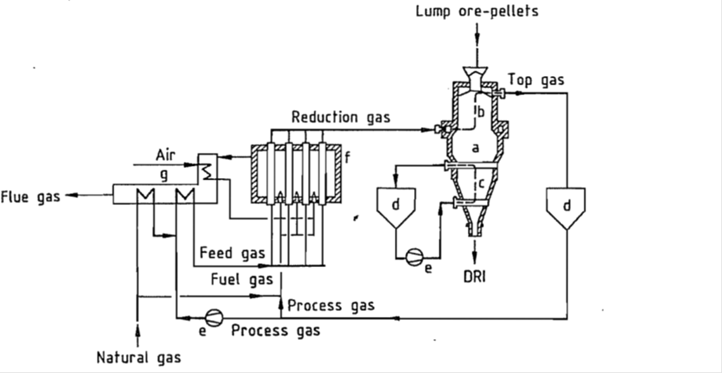
The exhaust gas (top gas) emitted from the top of the shaft furnace is cleaned and cooled by a wet scrubber (top gas scrubber) and recirculated for reuse. The top gas containing CO2 and H2O is pressurized by a compressor, mixed with natural gas, preheated and fed into a reformer furnace. The reformer furnace is provided with several hundreds of reformer tubes filled with nickel catalyst. Passing these tubes, the mixture of top gas and natural gas is reformed to produce reductant gas consisting of carbon monoxide and hydrogen. The reaction that occurs in the reformer tubes is as follows:
CH4+ CO2 = 2CO + 2H2
CH4+ H2O = CO + 3H2
2CH4 + O2 =2CO + 4H2
CO + H2O = CO2 + H2
CH4 = C(S) + 2H2
For more information please contact us.

