Steelmaking (Theory)
Steels are deformable alloys of iron with less than about 2%C and other elements. Steel contains C, Mn, Si, S and p .To make steels special properties, the steel is alloyed with other addition elements such as Cr ,Ni, Mo ,W ,Cu ,Nb and V. If the carbon content is higher than about 2%. It called pig iron.
The manufacture of iron in its pure form is a laboratory consuming and expensive process. Furthermore, compared to pure iron steels have substantially greater mechanical properties especially high strength.
Thermodynamics of steelmaking
Slag Systems
In the steelmaking process, slags are formed as products of the refining reaction by adding of flux such as lime. It is very important that the slag forms quickly, enabling the important reaction between metal and slag for example, desulfurization and dephosphorization. Two types of slags are much commended:
- Slag with Low phosphorus content
- Slag with high phosphorus content
Slag with Low phosphorus content
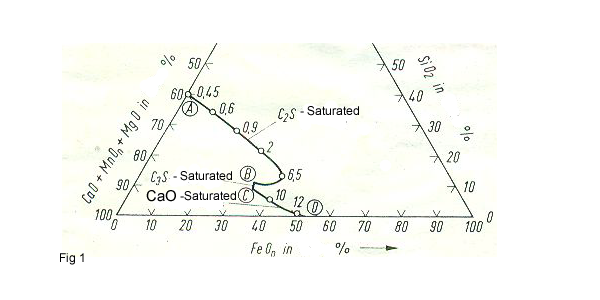
This system is FeO -CaO –SiO2,In general, the total of these three components is > 80% ,the remainder consisting mainly of MnO and smaller amounts of MgO.Al2O3,Cr2O3. Line AB shows the saturation surface of C2S = 2CaO.SiO2 and the line BC shows the saturation surface of C3S = 3CaO.SiO2 and the line CD shows the saturation surface of CaO. The slag of LD-convertor must be in the area of C2S and its FeO content should be about 25 -30 %.The slag content of arc furnace is at first in the area of FeO and in the end the slag content must reach the area between C2S and C3S.Because in this condition the desulfurization is carried out very well (fig1). The coefficient of sulphur distributions ȠS are shown as Numbers line on the mixed gap CaO.SiO2 (A-B-C-D).
Slag with high phosphorus content
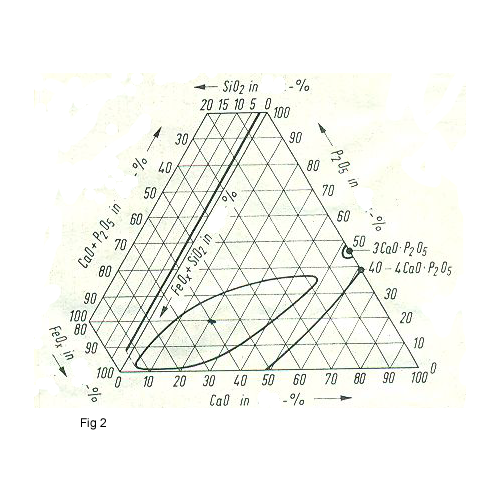
This system is FeO -CaO –P2O5 with 2% SiO2.This system can be used for the slag with high phosphorous .The slag must be reached the saturation area of CaO. The slags of Thomas process or LDAC lie in the area of CaO saturation and the line of mixed gap of system FeO- CaO –P2O5. Because in this condition the dephosphorization is carried out very well (fig2).
Metal – Slag Reactions
Refining Reaction
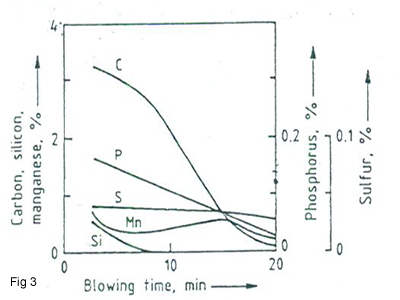
In the refining of pig iron to make steel, carbon , Si, Mn, P and other elements are removed by blowing the molten metal with oxygen. A typical concentration changes in the steel melt during oxygen blowing in a basic oxygen converter are shown in Figure 3. This figure shows how the impurities during blowing process will be decreased. The remove of impurities will be discussed as following:
Decarburization
Decarburization is occurred according the following reaction:
C + O = CO
The CO bubbles promote homogenization of the metal, and the elements H and N dissolved in the melt are picked up the bubbles as they ascend.
The equilibrium constant of the decarburization reaction is:
LogK = 1160/T + 2.003
At 1600 oC : [%C][%O] = 0.0025PCO
For more information please contact us.

