Theory of blast furnace
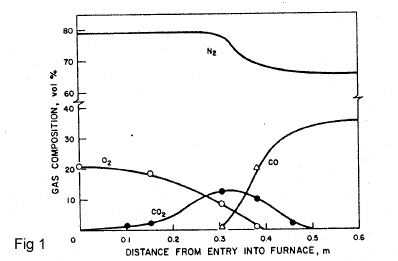
The main chemical reactions in the Iron-Blast furnace are oxidation of carbon by air in front of the tuyeres to give CO2 plus heat (fig 1):
C + O2 = CO2...(1)
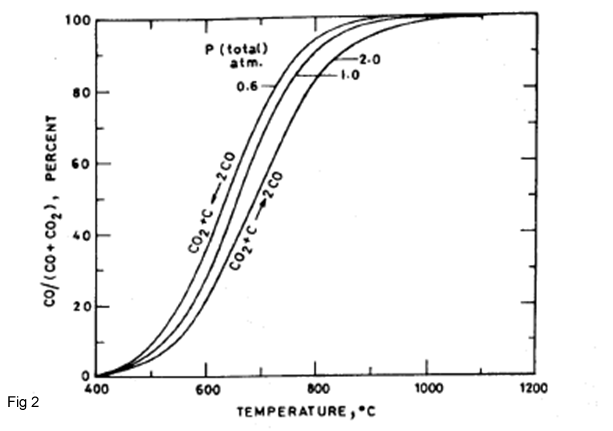
Endothermic reaction of the CO2 with carbon to produce CO is according to Boudouard reaction(fig 2):
CO2 + C = 2CO...(2)
There are there forms of iron oxide: hematite (Fe2O3), magnetite (Fe3O4) and wustite (FeO, non-stoichiometric compound).These oxides are reduced in stages. Their reaction with CO, the equilibrium CO/CO2 ratios and CO-utilization factors ȠCO at 900 0C are given below:
The CO-utilization factor is:
% ȠCO =100%CO2/%CO + %CO2The reactions, their CO/CO2 ratios and utilization factors at 900 0C are as following (table1):
3Fe2O3 + CO = 2Fe3O4 + CO2...(3)
Fe3O4 + CO = 3FeO + CO2...(4)
FeO + CO = Fe + CO2...(5)
Table 1
| Reactions | Equilibrium at 900 0C | Equilibrium at 900 0C |
| CO/CO2 | ȠCO,% | |
| 3Fe2O3 +CO = 2Fe3O4 + CO2 | 0 | ca.100 |
| Fe3O4 + CO = 3FeO + CO2 | 025 | 80 |
| FeO + CO = Fe + CO2 | 2.3 | 30 |
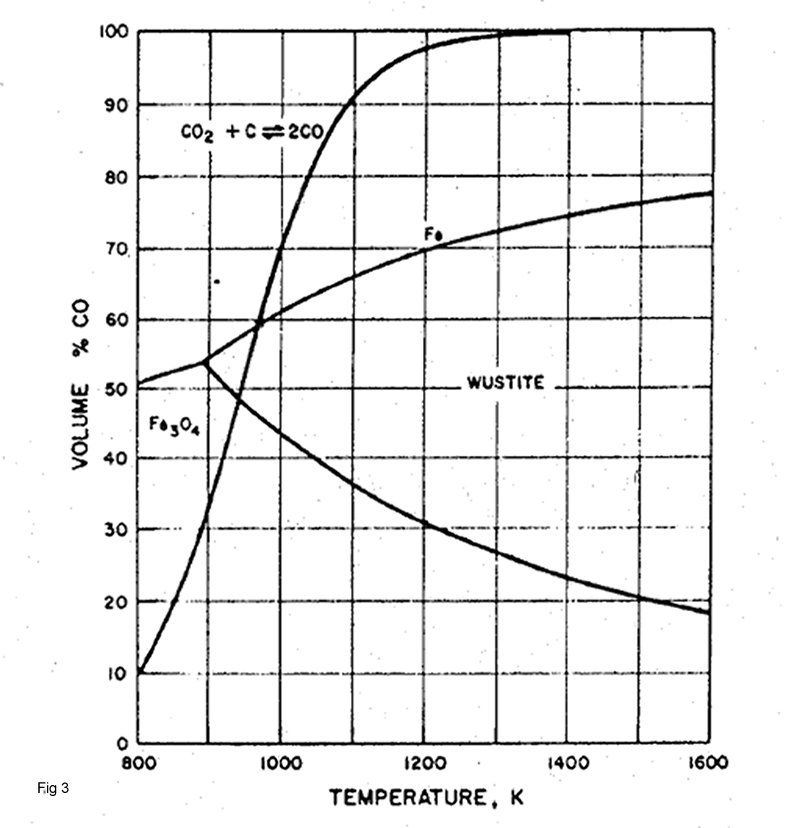
Equilibrium gas composition for Reactions 2,4 and 5are represented in Fig3.This figure shows that above 960 K the gas produced by the above reactions are sufficiently strong in CO to reduce wustite to iron but below this temperature it is not.Similarly the gas produced by Boudouard reaction can reduce to wustite at all temperatures above about 940 K and Fe2O3. to Fe3O4 at any temperature.
For more information please contact us.

