Deoxidizing copper
The quality of copper products greatly depends upon their electrical conductivity and mechanical properties, especial ductility.
The best ductility and toughness is obtained when the oxygen content of the copper is reduced to its minimum level of about less than 20ppm.
At higher percentages of oxygen ,copper could appear as Cu2O and in the presence of hydrogen ,it cause both macro-and microporosity.
The equilibrium of the hydrogen and oxygen reaction depends upon the temperature, pressure and the rate of activity of different phases.
The equilibrium between H and O (Figure1) shows that when the amount of oxygen reduces to a certain level, the amount of hydrogen increases. Hence, for complete deoxidizing, hydrogen should be removed. This can be done by element such as Li, P, Al, Mg and Ca, which act as deoxidizing agents by effectively removing the hydrogen from melt. However Vacuum or inert gas melting for cathodic copper provides effective deoxidation process.
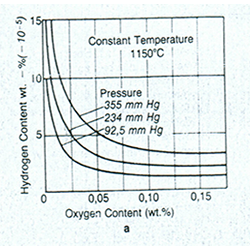

Figure 1 Equillibrium between hydrogen and oxygen of molten copper at (a)1150 oC with variation of presure.and (b) 92.5 mmHg with variation of temperture

