Defects
The crystals structure is not always perfect and in many structures there are some defects (imperfection) .There are several different kinds of defects that can be found in metallic crystals. The defects are grouped as following:
- Point defects (vacancy): zero dimension, a place where an atom is missing in the structure(Fig 1).
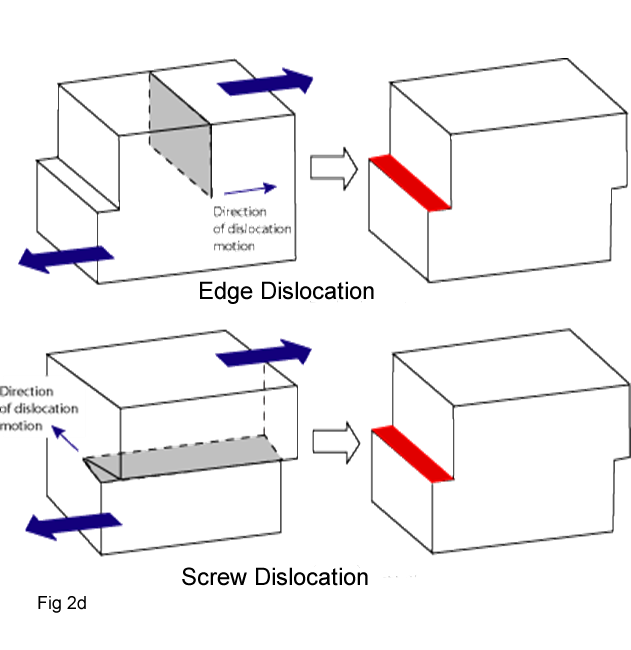
- Line defect: called dislocation is one dimension; it runs somewhat like a string through the crystal. A dislocation is the result of one atom or group of atoms being pulled slightly out of position with respect to perfect crystal packing (Fig 2a).There are three types of dislocations: edge dislocations, screw dislocations, and a combination of these two, termed mixed dislocations
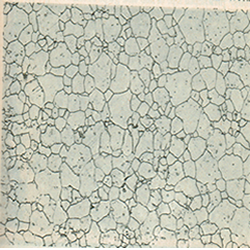
- Grain boundary defects: two dimensions, it is a two-dimensional interface between two different crystal grains in a solid sample. Since the two crystallites have in general different orientation, the structures do not match up exactly at the interface(Fig 3).
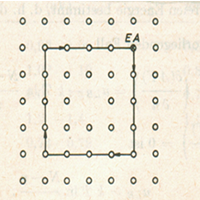
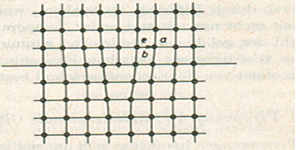
An edge dislocation(⊥) occurs when a single atomic plane does not extend completely through the lattice. The termination of this half-plane of atoms creates a defect line (dislocation line) in the lattice (Figure 2b). Edge dislocations can be quantified using a vector called the Burger's vector, b, which represents the relative atomic displacement in the lattice due to the dislocation(Figure 2b)
Screw dislocation occurs when the Burger's vector is parallel to the dislocation line(Fig.2d)This type of defect is called a screw dislocation because the atomic structure that results is similar to a screw.