Heat treatment
By the phase diagram, the microstructures of alloys that were allowed to solidify by slow cooling were considered. Therefore it is possible to modify the microstructure of an alloy by subjecting it to various thermal treatments. Heat-treating is a term used to describe all of the controlled heating and cooling operations performed on a material in the solid state for the purpose of altering its microstructure and/or properties. The focus of this discussion are metals but heat-treatment is also used on ceramics and composites to modify their properties.
The major objectives of different kind of heat treatment are as following:
- Increase the strength or hardness of material
- Increase the toughness of material
- Soften the material for improved workability
Different metals respond to treatment at different temperatures. Each metal has a specific chemical composition, so changes in physical and structural properties take place at different, critical temperatures. Even small percentages of elements in the metal composition, such as carbon, will greatly determine the temperature, time, method and rate of cooling that needs to be used in the heat treating process. Depending on the thermal treatment used, the atomic structure and/or microstructure of a material may change due to movement of dislocations, an increase or decrease in solubility of atoms, an increase in grain size, the formation of new grains of the same or different phase, a change in the crystal structure, and others mechanisms. Therefore there are many ways in which metals are heat treated. One method is used for most nonferrous alloys is Precipitation Hardening.
Precipitation Hardening
Precipitation hardening, or age hardening, provides one of the most widely used mechanisms for the strengthening of metal alloys. The precipitate particles act as obstacles to dislocation movement and thereby strengthen the heat-treated alloys. Many aluminium based alloys, copper-tin, certain steels, nickel based super-alloys and titanium alloys can be strengthened by age hardening processes.
Precipitation Hardening is occurred in 3 stages as following:
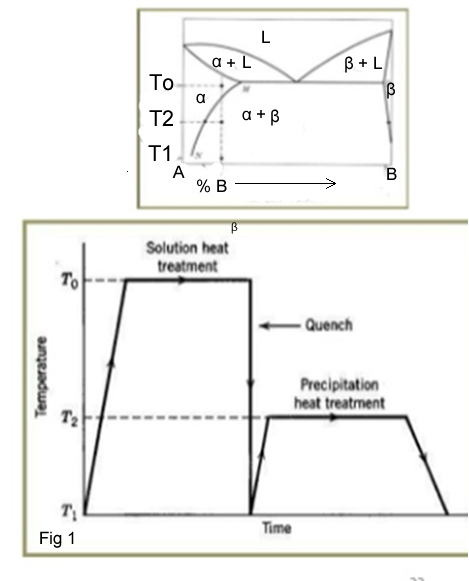
- Solid Solution:In this stage the alloy is heated to a temperature above the solvus line into the- phase and held for a period sufficient to dissolve the β-phase.
- Quenching :In this stage the alloy is quenched to room temperature or in water to create a supersaturated solid solution
- Precipitation Treatment:In this stage the alloy is heated to a temperature below Ts to cause precipitation of fine particles of β-phase(fig 1)