Precipitation hardening
Precipitation hardening, or age hardening, provides one of the most widely used mechanisms for the strengthening of metal alloys. The importance of theoretical suggestion for the development of new alloys is clear from the historical record. At the end of the 19th century, cast iron was the only important commercial alloy not already known to western technology at the time of the Romans. When age hardening of aluminium was discovered accidentally by Wilm, during the years 1903 -1911, it quickly became an important commercial alloy under the trade name Duralumin.
The strength and hardness of some metal alloys may be enhanced by the formation of extremely small uniformly dispersed second-phase particles within the original phase matrix in a process known as precipitation or age hardening. The precipitate particles act as obstacles to dislocation movement and thereby strengthen the heat-treated alloys. Many aluminium based alloys, copper-tin, certain steels, nickel based super-alloys and titanium alloys can be strengthened by age hardening processes.
In order for an alloy system to be able to be precipitation-strengthened, there must be a terminal solid solution that has a decreasing solid solubility as the temperature decreases. The Al-Cu (Duralumin is an aluminium alloy of 2XXX group) phase diagram shown in Figure 1 shows this type of decrease along the solvus between the α and α+θ regions. Consider a 96wt%Al – 4wt%Cu alloy which is chosen since there is a large degrease in the solid solubility of solid solution α in decreasing the temperature from 550°C to 25°C.If the alloy heated for several hours at 550 oC ,it will be single –phase structure .If it then is cooled quickly (e.g. quenched in water to 20 oC ,this single –phase structure will be retained, and thus a supersaturated solid solution is obtained. The alloy is then heated to an intermediate temperature and properties are measured as a function of time.
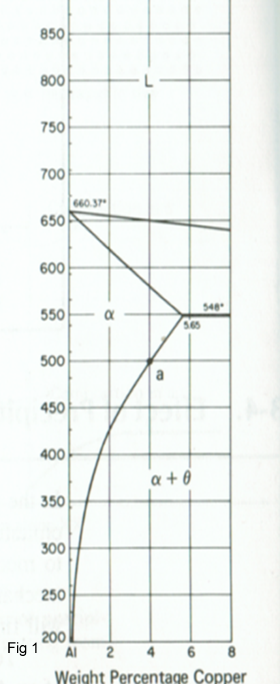
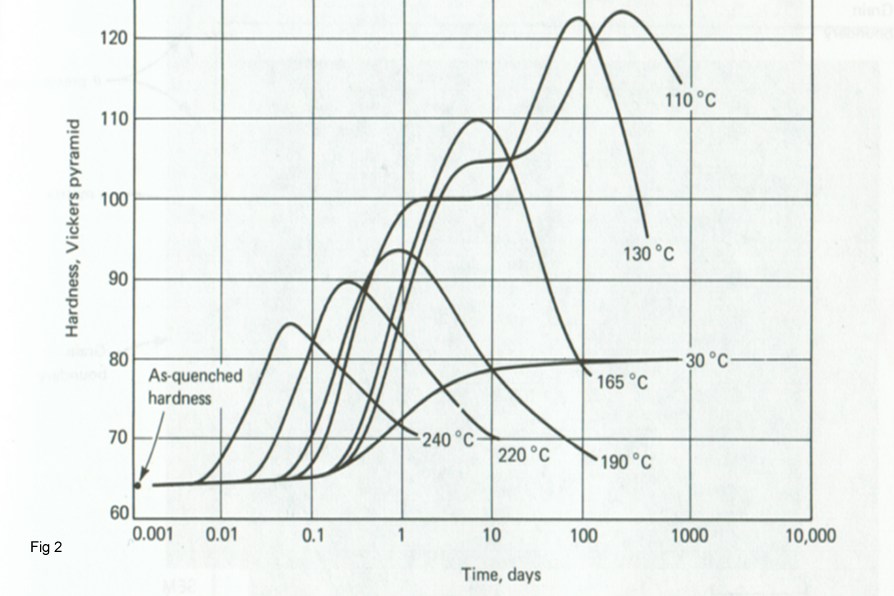
Figure 2 shows that the hardness and strength properties pass through a maximum and the curves are quite temperature depended. The maximum is associated with the precipitate attaining a critical size and distribution. When the particles become too coarse, they are too dispersed to be as effective in retarding dislocation motion, and hardness decreases.The strengthening which occurs is called precipitation hardening; If precipitation occurs at room temperature, the strengthening is called age Hardening.
Precipitation Mechanism
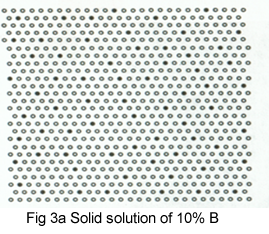
The process of precipitation is shown schematically in Figures 3a-e.Initially; B atoms segregate(fig3a) to form thin plates, GP zones (fig 3b). These thicken into plates in which B and A layers alternate, the θ'' structure (fig 3c). As the θ' 'precipitates increase in size, their crystal structure changes to give the θ' precipitate (fig3d).It is to know that this precipitate still has some interfaces on which atom position match those in the matrix, giving a coherent interface. The match is not perfect, however, and as the θ' increase in size, the elastic strains at the interface increase, eventually attaining a magnitude to generate a complex dislocation network .The strain is relieved and the interface becomes incoherent. Also, the crystal structure and chemical composition of θ' change to that θ (fig 3e).
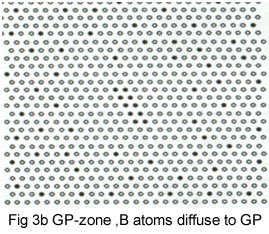

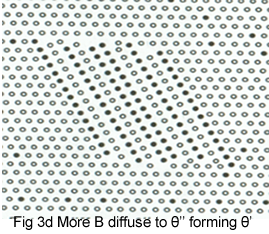
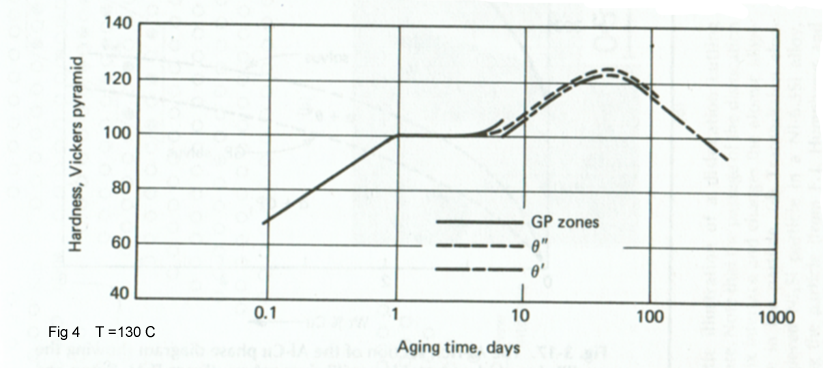
The relation of these structural changes to the hardness curve for an Al-4Cu alloy is shown in Fig 4.It is to note that the various stages overlap somewhat. The early stage of hardening is due to GP zone formation, and the additional hardening occurs as θ'' forms. The maximum corresponds to the presence of both θ' and θ''. The hardness decreases as θ' coarsens, and finally θ would be observed.