Bayer Process
Raw material for the electrolysis of aluminium is pure Al2O3.Pure Al2O3 will be produced by Bayer process. Bayer process was developed in 1887 by the Austrian chemist, Karl Josef Bayer. The commercial plant was begun in 1901 in East St. Louis. Illinois. The present world capacity is over 4x107 t/a.
The purpose of Bayer process is that boehmite, gibbsite, and diaspora can be dissolved in NaOH solutions under moderate conditions; the solubility of Al2O3 in NaOH is temperature dependent; most other components of the bauxite have less influence in the process; the silica that does dissolve subsequently forms a nearly insoluble compound. These feature let to form a sodium aluminate solution as follow (fig1):
Al(OH)3 + NaOH = NaAl(OH)4
The bauxite residue solids (red mud) are separated from the sodium aluminate solution.
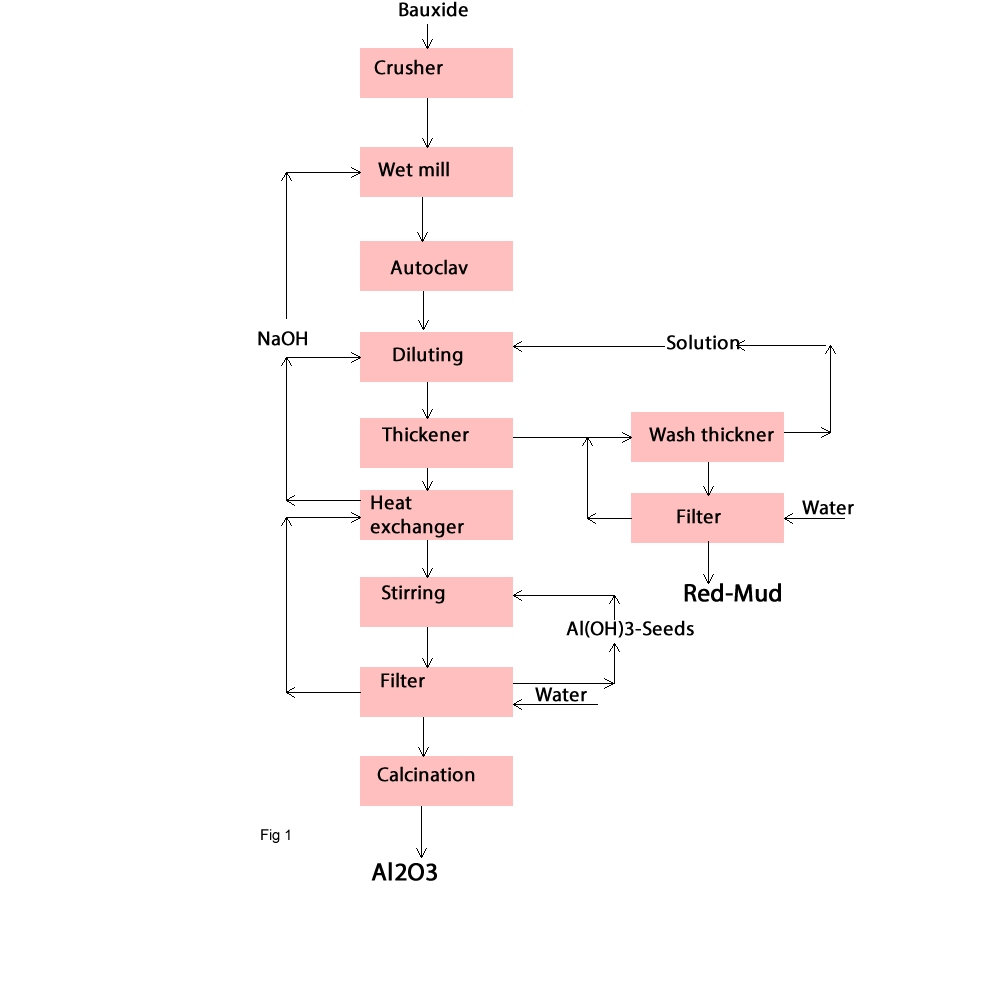
The solution, being free of solids, is cooled seeded with fine crystals of Al(OH)3 ;this causes the Al(OH)4 ions to decompose to Al(OH)3thereby reversing the reaction that previously had taken place digester. Again, the heat removed in cooling the solution is used to heat a colder steam in the process. After the precipitation reaction the coarse particles are washed and calcined to Al2O3 as following:
NaAl(OH)4 = Al(OH)3 + NaOH
2Al(OH)3 = Al2O3 + 3H2O
Bauxite preparation
The bauxite must be fine (< 0.15-2 cm) enough and the blending material must be analysed .Today bauxite will be stored in a surge tanks before it is pumped to digestion system. These agitated tanks are operated so that plant feed is uniformly mixed for several hours. Sometimes bauxites are dried to improved handling or washed to remove clay. In most modern plant today, the bauxite is mixed with a portion of the process solution and is ground as slurry. The slurry passed over screen or through cyclones .The fine particles will be participated in the process and the coarse ones being returned to the mills. In this process generally the ratio between solids and liquid is about 45 -55%.
Theory of Digestion
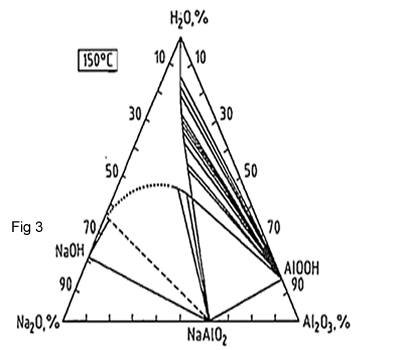
Figures 2,3 and 4 show the phase diagram Na2O-Al2O3 –H2O in different temperature. The solubility data of these diagrams show that Al2O3 concentration in the process solution can be increased by increasing the temperature, the NaOH concentration or both, therefor the operation conditions in plants can be vary widely. By choosing high temperature it results to use high pressure. There is also need for more heat-exchange equipment, which further increases the capital costs. If we use the high concentration, it results high productivity but it need dilation and additional cost for evaporation. Choosing digester conditions depends on balancing the physical factors with local economics and the designer's experience.