Electrolytic Production of Zinc
Standard electrode potential of zinc (Zn+2 + 2e = Zn) is - 0.76 volt. Due to standard electrode potential of hydrogen (2H+ + 2e = H2) is zero ,therefore a solution of zinc sulphate should not deposit zinc on electrolysis; hydrogen should be liberated instead zinc. Zinc deposition is only possible because of hydrogen overvoltage at the zinc electrode.
Study of Overvoltage
By electrolysis of metals which their electro potential standard are more than hydrogen during the process it is not occurred a serious problem but for the metals which their electro potential standard are smaller than hydrogen then hydrogen can be liberated instead metals .This problem will be occurred by electrolysis of zinc. The overvoltage is influenced by a number of factors which one of them is the current density. Figure 1 shows that the Zinc deposition is only possible because of the hydrogen overvoltage at the zinc electrode, which causes a voltage shift of magnitude approximately equivalent to the standard electro potential of zinc. This will be occurred by changing the current densities. The effect of current density is expressed by Tafel equation:
∆ = a +b log i; i = current density, ∆ = overvoltage , a=constant but it depends on electrode b = constant = 0.05 – 0.15 but generally 0.1
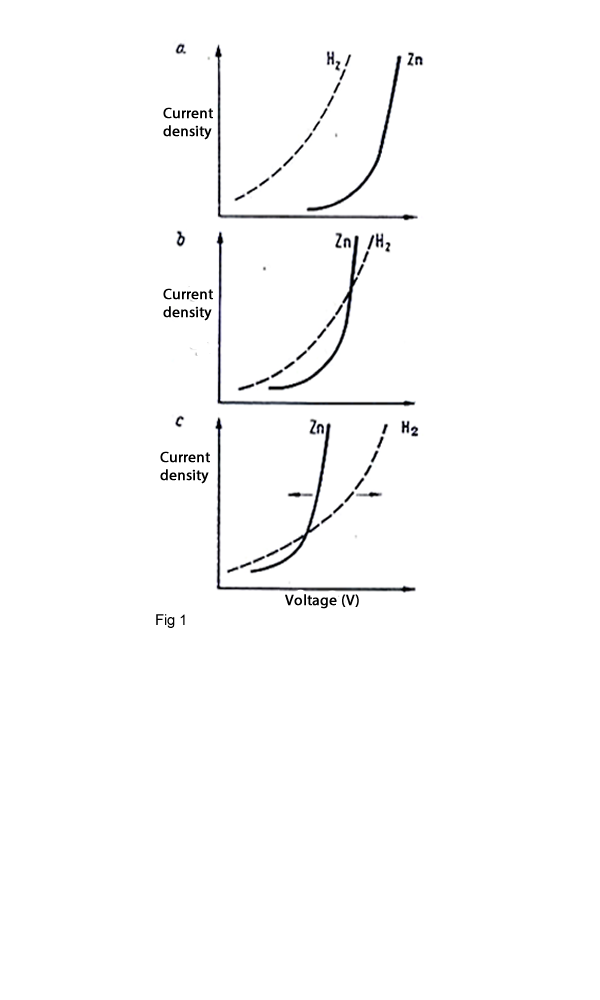
It decreases with increasing temperature, but as the conductivity decreases with decreasing temperature, the energy consumption increases. Based on these effects,two different typical methods of performing zinc electrolysis were designed. The first one works with low concentration of acid and a current density of 325 A/m2 and the second one works with high concentration and a current density of 850 A/m2.The process parameter widely used today with a current density of 400 – 600 A/m2 and a process temperature of 40 0C.The hydrogen overvoltage is also depended by the condition of the surface of the electrodes. Smooth surfaces lead to high over voltages, therefore in the zinc electrolysis industries the cathode will be stripped every one or two days but in copper electrolysis every two weeks.
Influence of Impurities
The influence of impurities can be fallen into several groups:
- Impurities those are more electronegative than Zinc such as K, Na, Ca, Mg, Al and Mn. These impurities don't caused any problem in the electrolysis but if their concentration are too high than the viscosity of the electrolyte increases and the diffusion processes in the boundary layer are disturbed, therefor a limit of 60 g/L is used.
- Impurities whose electrode potential lies between Zn2+ and H+ that can be fallen in two groups:
- Impurities such as Pb, Cd, Ti and Sn, the hydrogen overvoltage is higher than that of zinc. These impurities will be deposited on the cathode with zinc and the impurities of zinc will be increased, therefore the limit of these elements in the electrolyte must be controlled.
- Impurities such as Fe, Co and Ni, the overvoltage is lower than that of zinc. They are deposited on the cathode but can be redissolved by cell acid, therefore they decrease the current yield .For this reason the limit of these elements in the electrolyte must be controlled.
- Impurities those are more electropositive than zinc that can be fallen in two too groups:
- Impurities such as As, Sb, Te and Ge, these elements lead to a reduction in the overvoltage. They have a strongly effect on current yield. The limit of these elements must be controlled too.
- Impurities such as Cu and Ni, these elements lead to corrosion or redissolve of zinc in the cathode. The limit of these elements must be controlled too.
Reaction and Mechanism of Zinc Electrolysis
During electrolysis of zinc, several reactions can be occurred, but there is a general reaction as follow:
ZnSO4 + H2O = Zn + H2SO4 + ½ O2
The reaction on the anode and cathode are:
- Anode: 2H+ + ½ O2 + 2e = H2O , E0 =1.23 V
- Cathode: Zn2+ + (SO42-) +2e = Zn + (SO42-) , E0 =-0.76 V
The Sum of the voltage is: V = EA –EC + ∆A + ∆C + IR electrolyte + IR busbar = 1.23 – (-0.76) + < 0.5 + <0.7 +0.2 = 3.3 - 3.5 V
Regarding this equation for the electrolysis of Zinc a voltage of 3.3 – 3.5 V is necessary. This voltage is valid for a electrolyte with the following condition: Concentration of zinc = 50 – 60 g/L, concentration of H2SO4 = 150 -200 g/L, T = 30 – 38 0C Current density = 300 – 700 A/m2

