Matte smelting
The purpose of matte smelting is to melt concentrates or partially roasted concentrates at 1100-1250 0C to produce two separable liquid phases:
- Sulfide phase(matte): this phase consists of a mixture of Cu2S-FeS,which called matte (25-65 %Cu) with a density of about 5-5.5 gcm-3.The precious metal and another impurity are all soluble in this phase.
- Silicate Phase(slag): This phase consists of silicate, which has most Ca, Fe, Al and some impurity with a density of about 2.5-3 gcm-3.This phase is easy separable from matte.
The important purpose of matte smelting is to ensure sulphidization of all the copper present in the charge so that it enters in the matte phase.According to the following reaction to increase the activity of Cu2S, It must be decrease the activity of FeO. This is ensured by presence of FeS in the matte phase, which tends to sulphidize all the non- -sulphidic copper of the charge:
FeS + Cu2O = FeO + Cu2S
The equilibrium constant for equation:
K =aCu2S*aFeO/aCu2O*aFeSLog K= -∆Go/ 4.576T oK
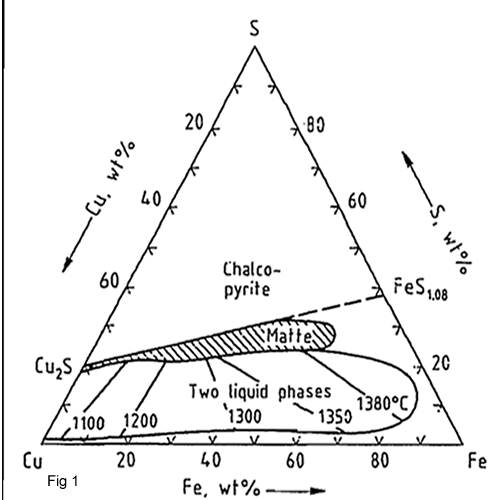
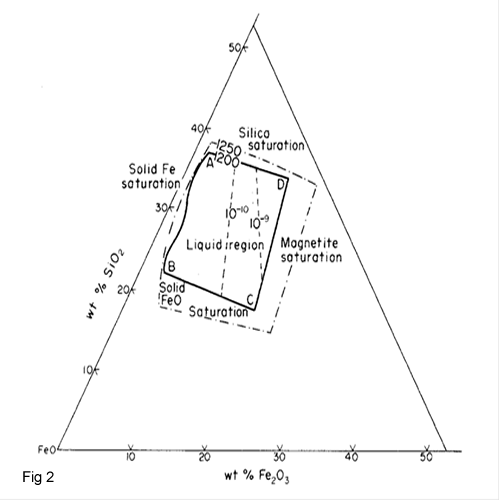
At smelting temperature about 1200 0C K is 10+4.This means that high value of K shows that Cu2O is almost completely sulphidized by FeS which is in agreement with practical experience The study of the Cu-Fe-S system shows (fig 1) that mattes can only exist in a narrow composition range between two phase Cu2S –FeS pseudo-binary which is hatched out in the figure 1.Slags from copper matte smelting contain 30-40 % Fe in the form oxides and about the same percentage of SiO2.mostly as iron II silicate. Such slags can be considered as complex oxides in the CaO-FeO-SiO2 system but because of the relatively low CaO content of most slags. The diagram in system FeO-Fe2O3-SiO2 is important to find the ideal temperature. Base on the figure 2, the interior of region ABCD marks the composition range in which the slags are completely molten at smelting temperature (1200oC).The optimum of matte/slag separations in the process will be obtained under near saturation conditions when the slag contains 32-45 % SiO2, 10-22 % CaO and till 50 % FeO.
Technology of matte smelting
Matte can be produced using different type of furnaces. These types of furnace are as follow:
- Blast Furnace
- Reveberatory Furnace
- Electric Furnace
- Flash Furnace
- Noranda and Teniente Smelting
- Isa process

