Hydrometallurgical Zinc Production
Today about 80% of Zinc production will be carried out through hydrometallurgical process and followed with electrolyte process. But these processes have still some problems. One of the important problems is precipitation and removal of iron. During roasting process is produced the component ZnFe2O4 .This component is difficulty soluble in sulphuric acid. To get high rate of solubility for this component, it is necessary to use hot and high concentrate sulphuric acid. Iron will be precipitated as Fe (OH)3.This precipitation make some difficulty by filtration process, therefore in the industry sometimes are happy by a recovery of 80-88 % Zn. Science 1960 - 1965 some process was found to improve the Zinc leaching process. The first one was Jarosit Process. By this process the precipitation was easy to filter, but after using this process in industry scale, they found that the Zinc residue contains small amounts of soluble zinc and must be deposited in special waste disposal sites; therefore new process such as Goethite and Hematite process have been developed. The leaching process will be occurred as follow:
Roasting
Before leaching process Zinc sulphide concentrates are roasted in a multiple hearth or fluidized bed roaster at 900 – 1000 0C.The roasted zinc concentrate then contains 55-70% Zn,2% Sulphate and 0.3 % sulphide. Sometimes there are zinc silicate and ZnFe2O4 in the concentrate. These component with zinc, copper, cadmium, .. Sulphides are not soluble in sulphuric acid, therefore will be remain as residue.
Leaching of Zinc concentrate
Zinc sulphide concentrate is after roasting process the important row material for the leaching process by sulphuric acid. In the past the amount of sulphuric acid was very important for leaching process but today it is used occasionally the cell acid from the electrolyse process, therefore the amount of acid isn't an economical factor. Today the solution of neutral leaching process will be sent to purification process and the residue contains Fe will be treated with one of this process; Jarosit, Goethite and Hematite process.
Neutral leaching
In This process Zinc concentrates after roasting or calcination process is leached by a mixture of cell acid (150 -200 g H2SO4/L ) and the acid solution (ca.50 g H2SO4) produced during iron precipitation. Adjusting the pH of the solution to 5-5.2, causes the Iron precipitation as Fe (OH)3 .The impurities such as Fe, As, Sb, and Ge are reduced. Because precipitation of iron causes As, Ge and Zn to be adsorptively coprecipitated.KMnO4 and MnO2 will be used for adjusting the pH (fig1).
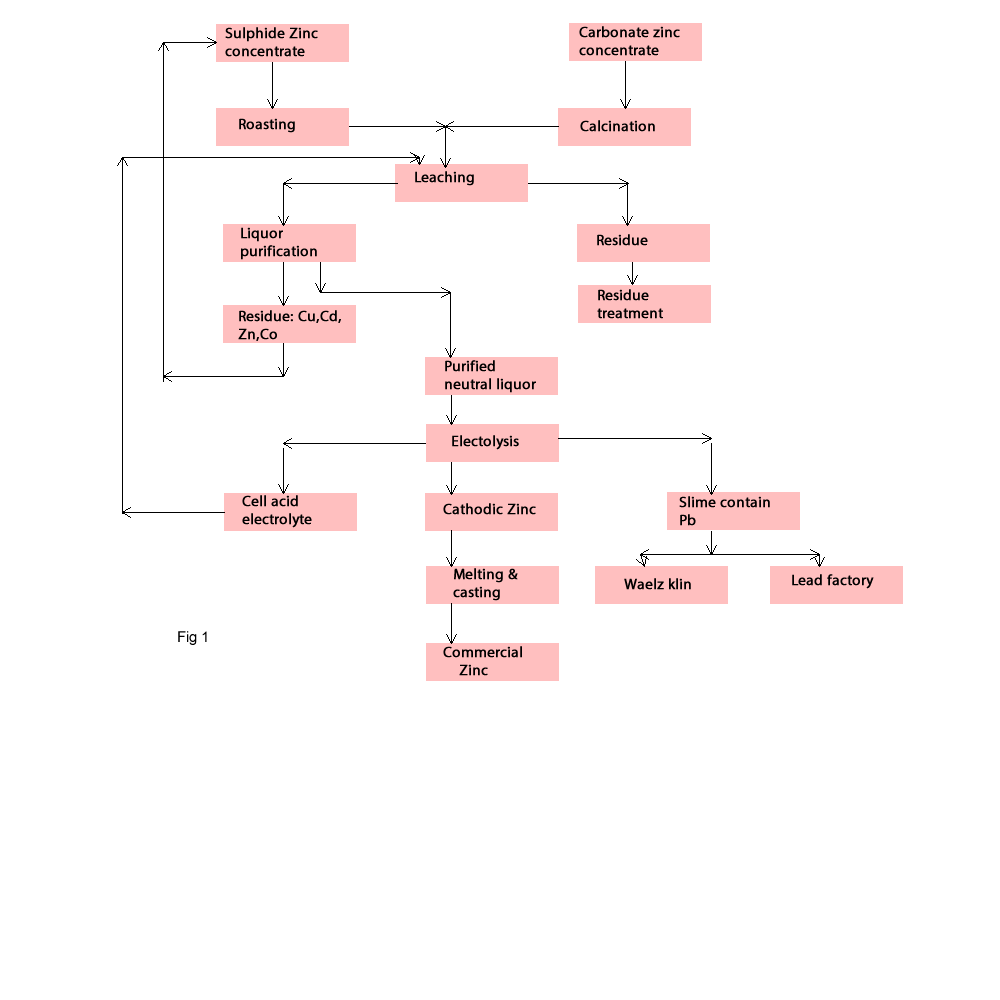
After leaching process the solution will be separated from precipitation Fe (OH)3 by filtration process. The solution will be sent to purification process and the precipitated Fe(OH)3 contains ZnFe2O4 will be leached with cell acid at 95 0C as hot acid leaching process. Then the iron will be removed by Jarosit or Goethite process and zinc will be recovered.
Jarosit method
After the hot acid leaching process the solution with precipitation is filtered .the precipitation contain Pb , Ag and Zn which have 5% Zn and Pb + Ag are separated from the solution and sent to Lead industry. The solution contain 80 g/L zinc, iron and another impurity such as As ,Sb ,Ge ,Ni and Co entered into preneutralization system. In this stage calcine powder (ZnO) will be added into the tank. After this process solution with precipitation will be filtered again. The precipitation sent to the hot acid leaching process and the solution entered into Jarosite precipitation process (fig 2).
In this stage alkali (Na+ or K+ ) or ammonium (NH4+) ions will be added to the solution regarding the following reaction:
6FeSO4+ 3/2O2+ 2NH4OH+ 7H2O = 2(NH4) Fe3(SO4)2(OH)6 + 2 H2SO4
In the Jarosite precipitation are an Fe (III) compound of the type X [Fe3(SO4)2(OH)6],where X represents H3O+ , Na+ , K+ or NH+4 Procedure conditions are as follow:
- pH = 1 to 1.5
- T= 180 0C by high acid concentration 60 – 90 gH2SO4/L or T= 95 0C by low acid concentration 5 – 10 gH2SO4/L
The lower temperature method is more widely used, because of the lower equipment costs.The jarosite residue contains 4 – 6% Zn and 25 – 30 % Fe.

