View of Lead Production
Lead minerals are difficult to leach with acid solution; therefore approximately 95-100% of the world's lead extraction methods are by pyrometallurgical process. The extraction consists of two different base processes:
- Roast Reduction process
- Roast Reaction process
- Boliden Electric
- Kivcet
- Boliden Kaldo
- Outokumpu
- QSL
- Isa Smelt
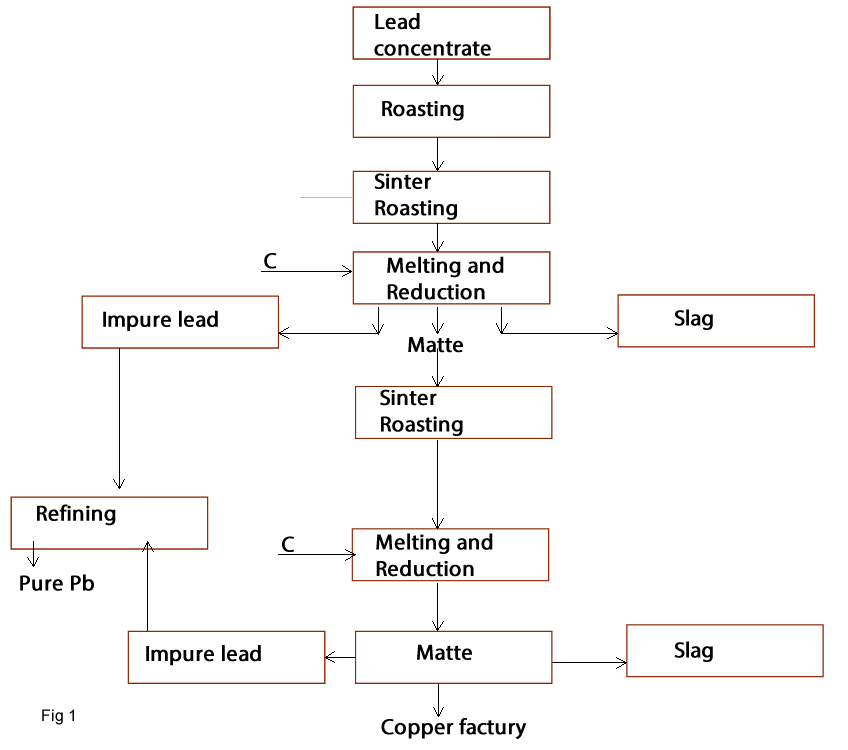
Roast Reduction process:
Lead ores or lead concentrate is roasted. This process is most a sintering oxidation to produce a suitable charge for using in the blast furnace. The reduction process will be done in the blast furnace using C and CO as reduction agent.By using these processes; impure lead is produced and it is refined to obtain pure lead. (fig1).
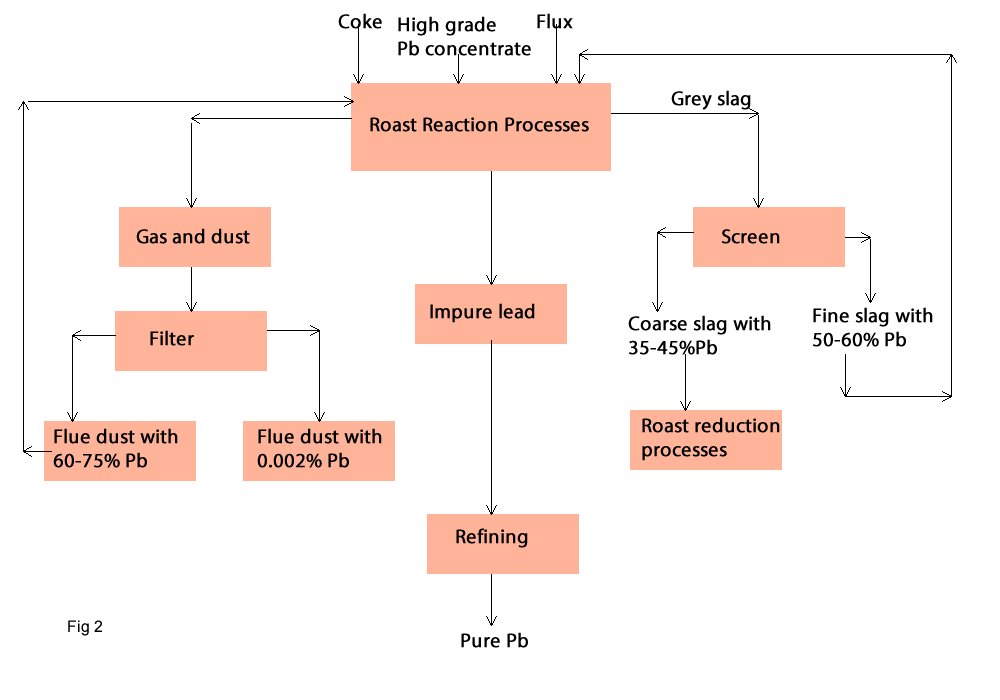
Roast Reaction Process:
In this process lead sulphide reacts with lead oxide or lead sulphate to form metallic lead and SO2 in the roast reaction. This direct smelting reduction will be carried out in one or two steps in one or two furnaces, but mostly in one furnace (fig2). Today this process is used for high grad concentrate therefore several processes were developed and employed in the industry such as:
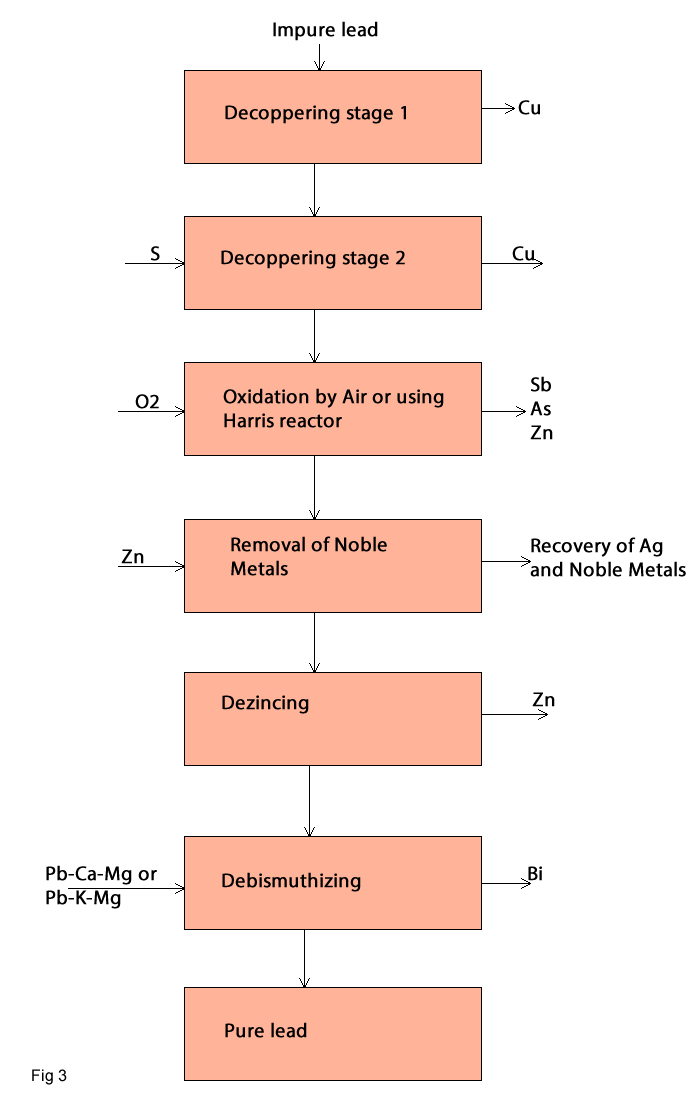
Refining
The melting process does not produce pure lead.The produced lead has 96-99% pb. To produce pure lead the refining process must be carried out.Figure 3 show the steps, which should be done to remove the impurities.