View of Aluminium Production
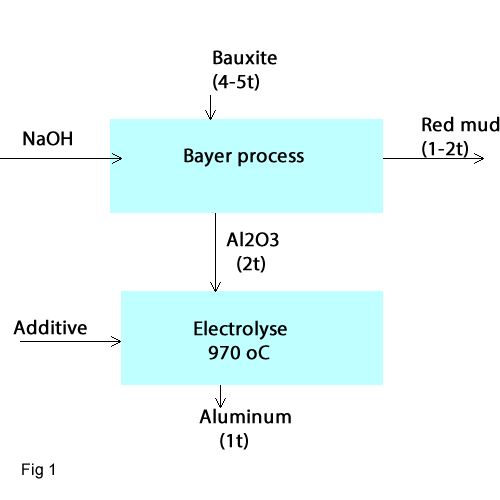
Nowadays Aluminium is produced in two stages. (fig1):
- Stage 1: In the Oxide industry pure Al2O3 is won from bauxite in an autoclave by a defined temperature and pressure using Bayer process.
- Stage2: During electrolytic aluminium processing pure Al2O3 is solved in molten cryolite and produced Aluminium
Stage 1
In this stage pure Al2O3 is produced via hydrometallurgical process. There are several leaching method as follow:
Alkali leaching
The alkali leaching is as follow:
- Bayer process
- Leaching of Bauxite with high silicate (SiO2)
- Pyrogen process; Leaching bauxite using NaOH as leaching agent
Acid leaching
- Leaching with H2SO4
- Melting and leaching
- Hall-method
- Hagland method
and........
The Bayer process is the important process which is used in the industries today ; therefore it will be studied in details later.
Bayer Process
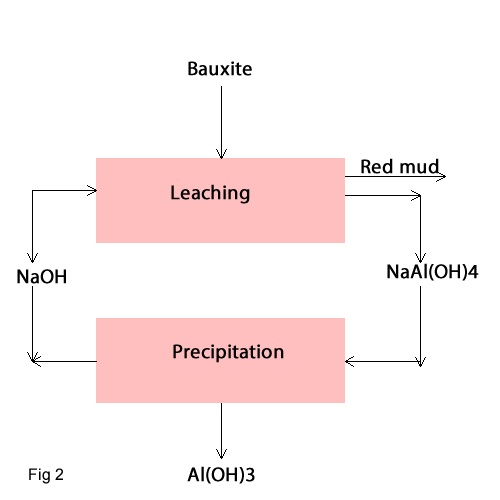
In this process; Leaching of Aluminium hydroxide in NaOH with 130-135 g Na2O/L at high temperature and presure is occurred
so
it produces NaAl(OH)4 solution and red mud as undouble material . Then when the solution is cooled
it produces Al(OH)3 base
the following reaction (fig2) :
:
NaOH + Al(OH)3 = NaAl(OH)4
NaAl(OH)4 = NaOH +Al(OH)3
The red mud will be removed.
Stage2
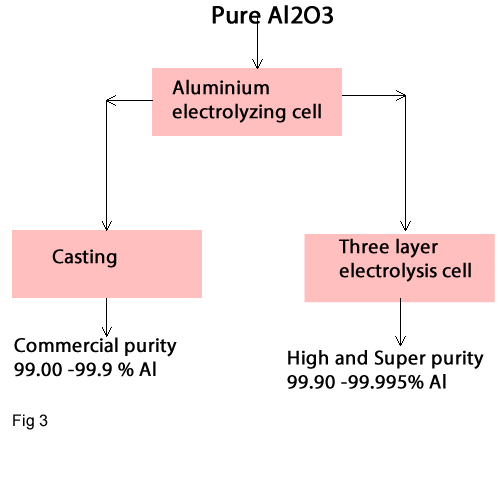
In this stage aluminium is produced from pure Al2O3 by electrical method. The product is commercial aluminium with a grade of about 99.00-99.9% Al. If Aluminium with high grade is needed, then the commercial aluminium is processed via three layer electrolysis to obtain a high or super purity of aluminium with a grade of 99.9 -99.995% Al (fig3)
Production of Aluminium via electrolysis
In this process, the pure Al2O3 is dissolved in molten cryolite and then Al2O3 via electrical current is ionised and its ions are free to move around. Aluminium ions are being reduced and collected to the bottom as carbon cathode. At the anode (positive electrode), oxygen is formed .The carbon anode is then oxidized by the oxygen
Refining of Aluminium via three layer electrolysis cell (Hoop's -method)
This process has three liquid layers. The lowest layer, called anode metal, receive the input of comercal aluminium to which about 30% Cu has been added to increase the specific gravity to 3.4 -3.7 g/cm3.The second layer is the molten aluminium (as electrolyte) with a specific gravity of about 2.7 -2.8 g/cm3.The third layer is the separated high-purity liquid aluminium with a specific gravity of 2.3 g/cm3.The produced aluminium has the high purity of a grade of 99.9 -99.995 %Al.