Electrolytic Reduction of Alumina
Aluminium will be produced by the process-electrolysis of fused salts, the use of cryolite as an electrolyte( flux) to dissolve alumina ,and the use of carbon electrodes.
Raw materials
- Carbon: In the electrolysis of aluminium production part of the energy for reducing alumina is supplied as electricity and parts comes from consumption of the carbon anode. About 0.4-0.5 kg of anode is consumed for one kg Aluminium production. Carbon is used as the cathode lining too.
- Aluminium Oxide: About 1.9 -1.95 kg of Al2O3 (alumina) are used for production one kg aluminium.
- Electrolyte Materials: The electrolyte is a solution of aluminium oxide in cryolite. Cryolite comprises more than 75% of the electrolyte which contains calcium fluoride (4-8%) ,aluminium fluoride (5- 15%) alumina (1- 6%) and sometimes lithium fluoride (0-5%) and magnesium fluoride (0-5%).These additives lower operating temperature and increase current efficiency.
Theoretical Principles of Fusion Electrolysis
Electrolytes
The mineral cryolite is a component of 3NaF.AlF3 (Na3AlF6) and has a melting point of about 1010 oC. Synthetic cryolite can be produced by the following reaction:
6HF + 2NaOH = Na3AlF6 + 4H2O
Cyrolite is produced directly in reduction cell by reaction of the soda impurity in the feed alumina with added aluminium fluoride as following:
3Na2O + 4ALF3 = 2Na3AlF6 + Al2O3
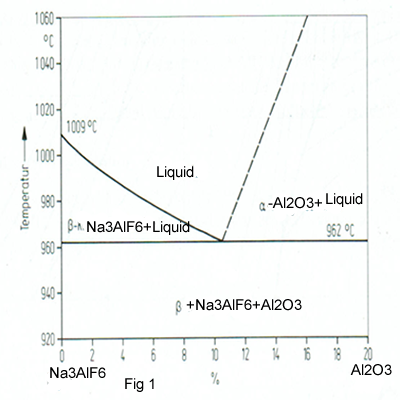
There are a compound with 40% AlF3 and 60% NaF and called Cyrolite (Na3AlF6).It melted at about 1000 oC.Al2O3 has a melt point about 2150oC.Figure1shows that about 10-11 %Al2O3 is soluble at about 962 oC in Cyrolite but this solubility will be reduced by the additive such as AlF3,CaF2 and Li3AlF6.
During electrolysis of aluminium several additives will be used, they have different influence of the physical properties of the process.

