View of Zinc Production
Zinc mineral after calcination or roasting process is easily soluble in acid especially sulphuric acid, therefore today 70-80 % of Zinc productions are carried out from hydrometallurgical process. But there are two methods for the production of zinc from its ore..
- Pyrometallurgical process
- Hydrometallurgical process
Pyrometallurgy
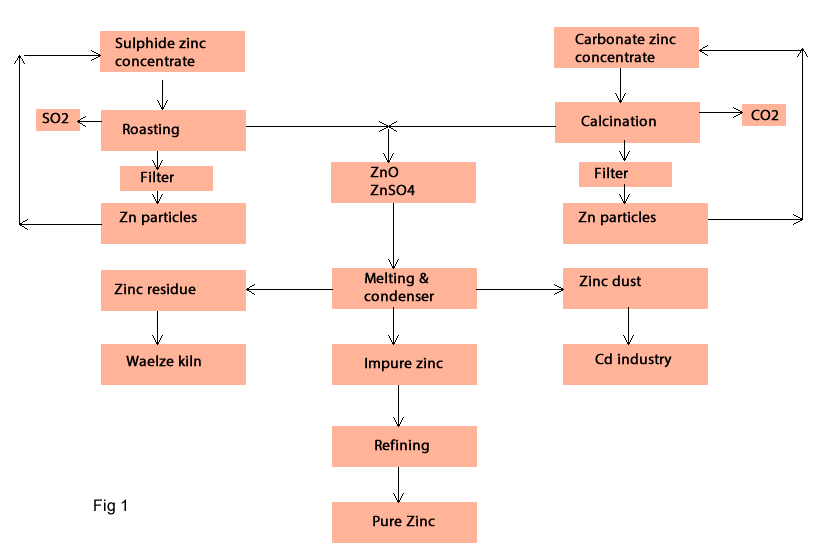
In pyrometallurgy method the sulphide concentrate must be prepared for metal extraction by roasting process. The oxide concentrate (carbonate) also need to be prepare for metal extraction by calcination process. After this process the zinc -oxide or zinc -sulphate is reduced at 1200 – 1300 0C by C or CO to produce zinc vapour; then the zinc is condensed at 550-600 0C in a condenser to produce zinc metal. The produced zinc is impure and needs to be refined by New Jersey process. The refined zinc is very pure and is used in industry (fig1).
Hydrometallurgy
In hydrometallurgy Process the zinc concentrate, after roasting or calcination, is leached with sulphuric acid. Zinc-sulphate after leaching process isn't pure and must be refined. The refining process takes place in several tanks. Cementation with zinc powder is the most important step in refining process. After refining process sulphate solution with 120 g/L Zn is produced. This solution is electrolysed to produce canthodic zinc with more than 99.9 % Zn (fig2).


