View of Copper Production
Copper is produced by two different methods:
- Pyrometallurgy
- Hydrometallurgy
- Concentration by flotation: In this method copper sulphide minerals with about 05-2% Cu are made to become selectively attached to air bubbles rising through an aqueous pulp of ores; this process is aided by adding reagents which is adsorbed the copper minerals hydrophobic, while the gangue mineral are left. The product called concentrate with about 20-30% Cu.
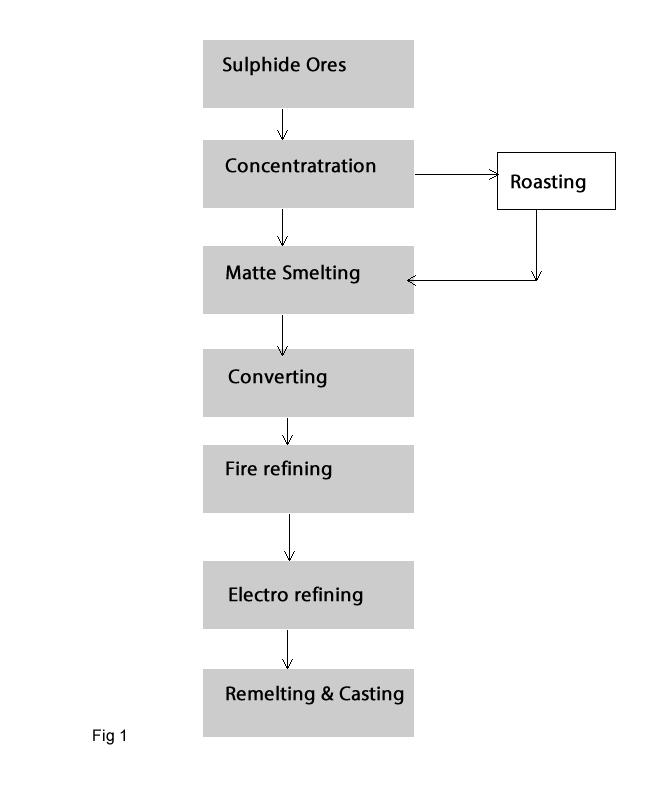
- Roasting : it is an optional step by production of copper. If the sulphur in the mineral is enough for matte smelting process the roasting process will be not used and the process is carried out only by high sulphur content in the mineral. Roasting consists of partially oxidizing the sulphides of concentrate and removing sulphur from them as SO2.
- Matte smelting: this is an enrichment process of copper mineral with sulphur. In this step copper mineral will be smelted at about 1200 oC in the furnace to produce a phase of Cu2S and FeS which called Matte. During this process the grade of copper will be increased to about 30-60% Cu. Several furnaces are used for matte smelting process such as blast, reverberatory, electric, flash or Isa furnaces.
- Blister copper: In this step Converting of Matte to blister copper is occurred. This process consists of oxidizing the liquid matte from smelting. Converting removes some impurities specially Fe and S from the matte and produce Blister copper with about 98-99% Cu.
- Fire refining of blister copper: Electrorefinning process needs a strong, flat, thin anodes with the cathodes in the refining cells, but this cannot be obtained by directly casting of blister copper due to the oxygen and sulphur content in the blister. Therefore in this process the oxygen and sulphur is decreased and the molten metal is cast as anode. .
- Electrolyse of copper: Electrorefining of fire refined (tough-pitch) copper is for the production of copper cathode. Electrorefining of copper consists of the electrochemical dissolution of copper from the impure anodes and producing pure copper cathode with a grade of more than 99.9% Cu.
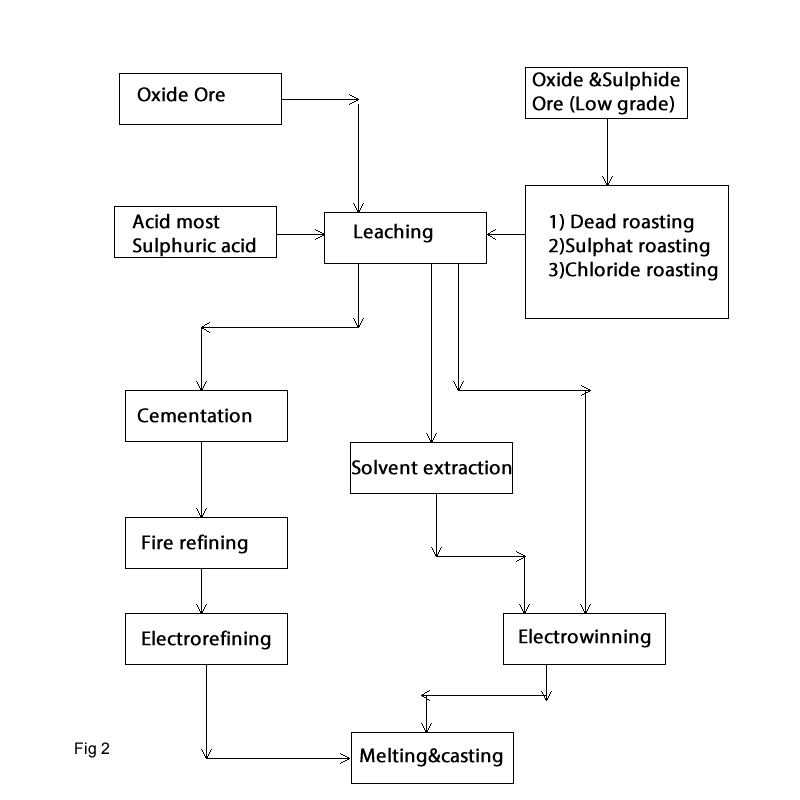
- Leaching
- Heap leaching for low grade ores less than about 1%Cu
- Vat leaching for rich to about 2% Cu
- Agitation leaching for oxide concentrates or roasted sulphides with about 20-30%Cu
- Pressure leaching for sulphide mineral or concentrate.
- Extraction of copper from the solution
- For Heap leaching:
- For Vat and Agitation process:
Pyrometallurgy:
Approximately 80-90% of the world's primary copper originates in sulphide ores; therefore the vast majority of the extraction of copper is by pyrometallurgical process. The extraction consists of the following 6 steps (Fig 1):
Hydrometallurgy:
Oxide or Oxidized copper ores and low-grade oxide and sulphide ores and wastes material are extracted by Hydrometallurgical process. The extraction consists of the following steps (Fig 2):
In process copper mineral or copper concentrate is leached mostly with sulphuric acid; then copper will won with cementation or electrowinning process. There are several leaching methods for the hydrometallurgy of copper:
The extraction of copper from the solution carried out via following process:
cementation: in this process iron scrap is added to the solution and copper is precipitated according the following reaction: Cu++ +Feo = Fe++ + Cuo It produces Impure copper which is used in converter as charge material. Another option is to increase the copper in the solution via Solvent extraction method and then copper will be extracted through Electrowinning process.
The copper is extracted via Electrowinning process. Electrowinning process is similar to electrorefining except that anode is insoluble material such as antimonial lead. In this process copper is produced as cathode copper with a grade of 99.5% Cu