Fe-C Diagram
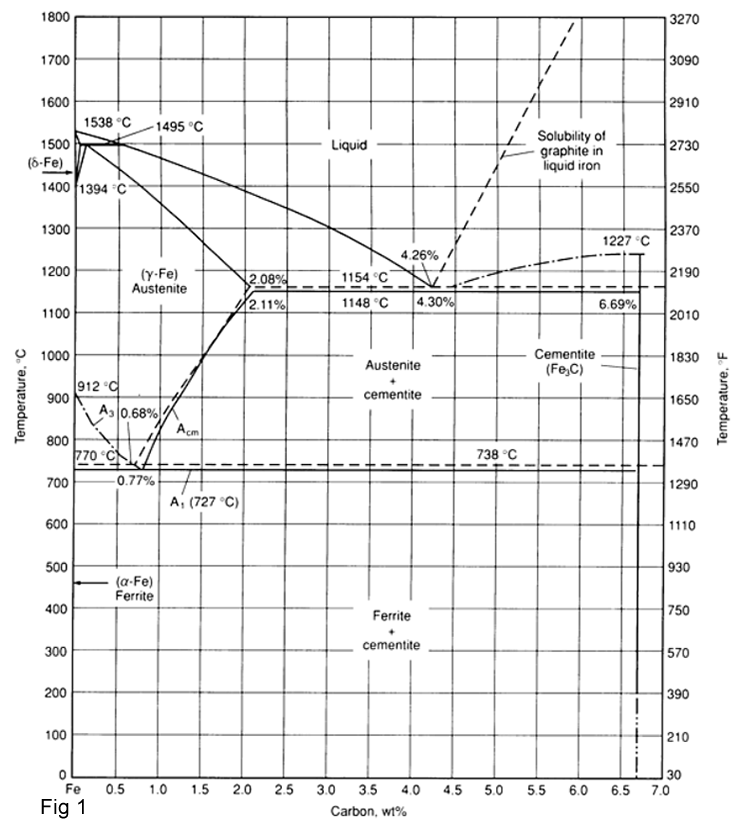
The Fe-C phase diagram is a fairly complex one, but in this part it will only consider the important parts (fig 1).
Phases
There are several phases in Fe–Fe3 Phase Diagram such as:
- α-ferrite - solid solution of C in BCC Fe, it has Stable form of iron at room temperature. The maximum solubility of C is 0.022 wt%.It transforms to FCC γ-austenite at 912 °C.
- γ-austenite - solid solution of C in FCC Fe. The maximum solubility of C is 2.14 wt %. It transforms to BCC δ-ferrite at 1395 °C. It is not stable below the eutectic temperature (727 ° C) unless cooled rapidly.
- δ-ferrite solid solution of C in BCC Fe. It has the same structure as α-ferrite. It is stable only at high T, above 1394 °C. It melts at 1538 °C.
- Fe3C (iron carbide or cementite). This intermetallic compound is metastable, it remains as a compound indefinitely at room T, but decomposes (very slowly, within several years) into α-Fe and C (graphite) at 650 - 700 °C.
- Fe-C liquid solution.
Invariant Reaction in the Fe-Fe3C Diagram
The Fe3C phase diagram has three invariant reaction, each of which occurs at constant temperature and involves three phases. These reactions are peritectic, eutectic and eutectoid.
- Peritectic Reaction: At the peritectic reaction point, liquid of 0.53% C combines with δ-ferrite of 0.09%C to produce γ-austenite of 0.17%C.The reaction is as following:
Liquid + δ-ferrite = γ-austenite T=1495 °C
- Eutectic Reaction: At the eutectic reaction point, liquid of 4.3% C decomposes to produce γ-austenite with 2.08% C and the intermetallic component Fe3C (cementite) which contain 6.67 %C. Eutectic of austenite and cementite is known as ledeburite The reaction is as following:
Liquid = γ-austenite + Fe3C T=1148 °C
- Eutectoid Reaction: At the eutectoid reaction point,solid austenite of 0.8% C decomposes into α-ferrite with 0.02% C and cementite with 6.67% C . The reaction is as following:
γ-austenite = α-ferrite + Fe3C
Eutectoid, Hypoeutectoid and Hypereutectoid
A carbon steel containing 0.8% C is called a eutectoid steel since the eutectoid transformation of austenite to cementite and ferrite occurs at this composition. If the carbon content of the steel is less than 0.8%C, it is called Hypoeutectoid steel .Most steels produced commercially are Hypoeutectoid steel.
Steels containing more than 0.8%C are called Hypereutectoid steels. Hypereutectoid steels with up to about 1.2%C are produced commercially.When the carbon content of steel is more than 1.2% ,the steel will be very brittle and thus few steels are made more than 1.2%C.For Increasing the strength of steels, other alloying elements are added which increase the strength as well maintain ductility and toughness.
Slow Cooling of Carbon Steels
Eutectoid Carbon Steel
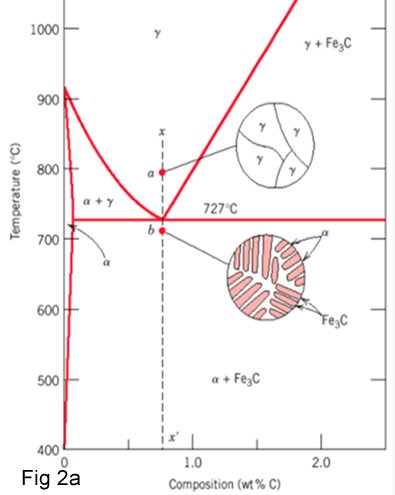
If a sample of Fe-alloy with 0.8% C is heated to about 750 °C for sufficient time. Its structure becomes homogenous austenite.
If there is any undercooling, the entire structure will be transformed from austenite to lamellar structure of alternate plate of α-ferrite and cementite (Fe3C) (Fig2a-2b).Since this eutectoid structure as seen in optical microscope resembles mother of pearI, it has been named pearlite.
Fig 2b shows the microstructure of lamellar eutectoid Pearlite .In the micrograph, dark regions are cementite and bright regions are ferrite.
For more information, please contact us

